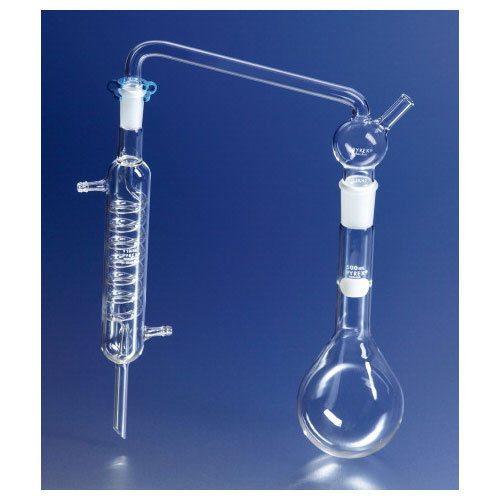
Distillation Set for Determination of Ammonia
Distillation Set for Determination of Ammonia
The set includes the glass parts:
Includes:
- 500mL round flask with long neck
- Leibig condenser
- Adapter with two heads
Ideal for determining Nitrogen in organic compounds.
Digestion and Distillation stages can be performed in this apparatus.
The inlet tube allows permanganate solution to be introduced after distillation.


 Labdisc
Labdisc Botzees
Botzees Edison
Edison Telepresence Robot
Telepresence Robot DOBOT
DOBOT Keyestudio
Keyestudio Fischertechnik
Fischertechnik