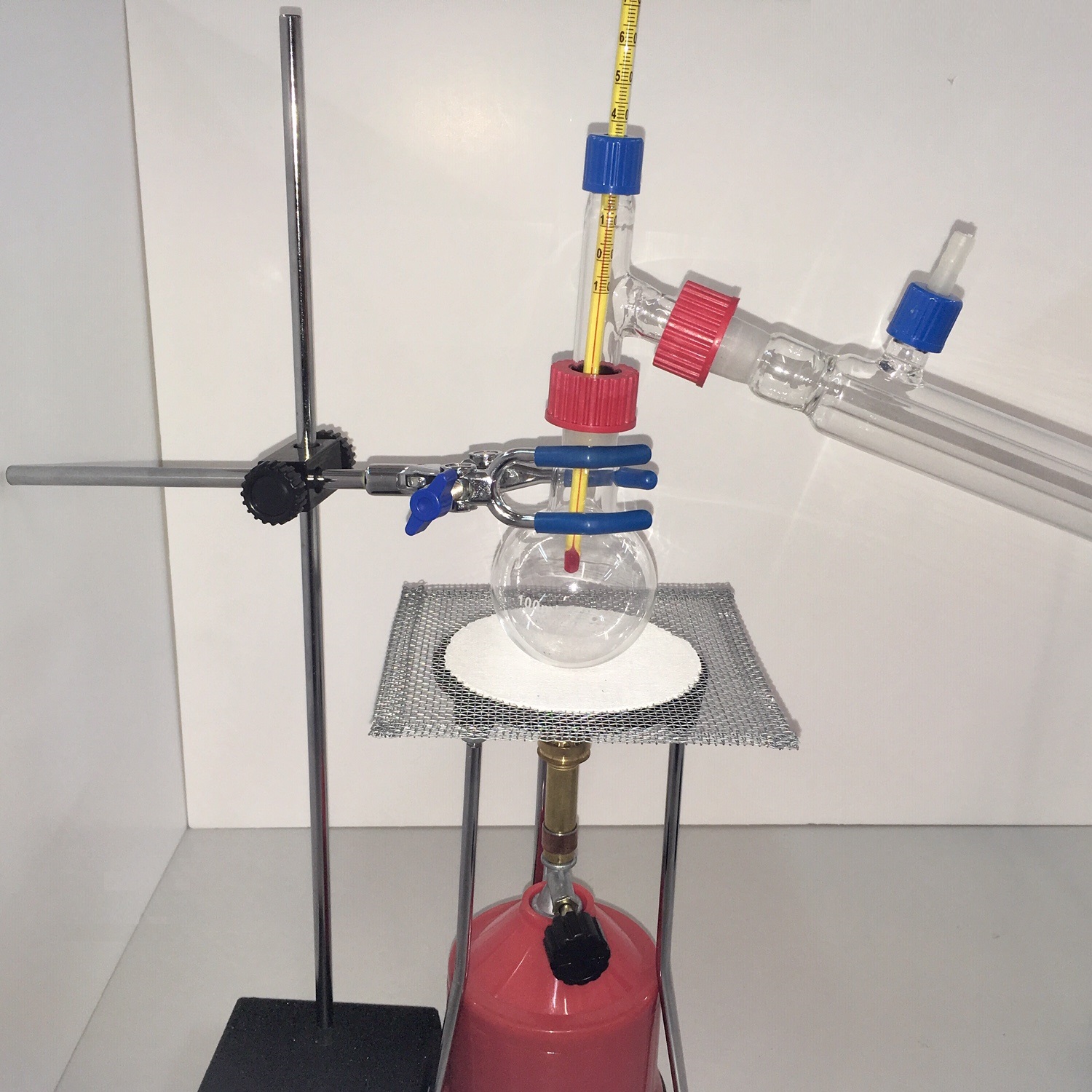
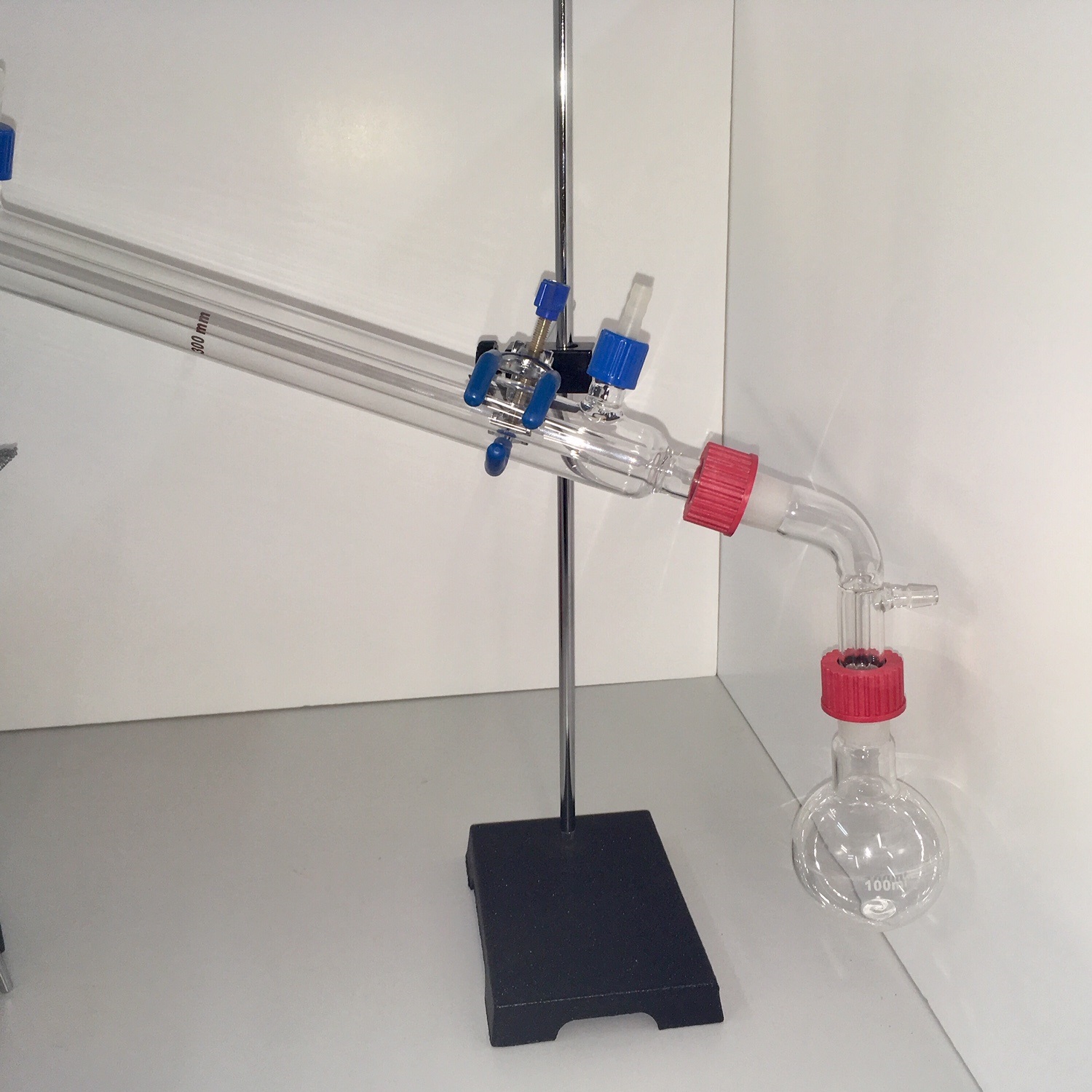
Distillation Set with Bunsen
Distillation, or classical distillation, is the process of separating the components or substances from a liquid mixture by using selective boiling and condensation. Dry distillation is the heating of solid materials to produce gaseous products (which may condense into liquids or solids). Dry distillation may involve chemical changes such as destructive distillation or cracking and is not discussed under this article. Distillation may result in essentially complete separation (nearly pure components), or it may be a partial separation that increases the concentration of selected components in the mixture. In either case, the process exploits differences in the relative volatility of the mixture’s components. In industrial applications, distillation is a unit operation of practically universal importance, but it is a physical separation process, not a chemical reaction.
Applications
The application of distillation can roughly be divided into four groups: laboratory scale, industrial distillation, distillation of herbs for perfumery and medicinals (herbal distillate), and food processing. The latter two are distinctively different from the former two in that distillation is not used as a true purification method but more to transfer all volatiles from the source materials to the distillate in the processing of beverages and herbs.
The main difference between laboratory scale distillation and industrial distillation are that laboratory scale distillation is often performed on a batch basis, whereas industrial distillation often occurs continuously. In batch distillation, the composition of the source material, the vapors of the distilling compounds, and the distillate change during the distillation. In batch distillation, a still is charged (supplied) with a batch of feed mixture, which is then separated into its component fractions, which are collected sequentially from most volatile to less volatile, with the bottoms – remaining least or non-volatile fraction – removed at the end. The still can then be recharged and the process repeated.



















 Labdisc
Labdisc Botzees
Botzees Edison
Edison Telepresence Robot
Telepresence Robot DOBOT
DOBOT Keyestudio
Keyestudio Fischertechnik
Fischertechnik