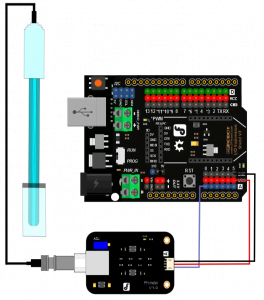
Gravity: Analog pH Sensor/Meter Pro Kit for Arduino
Gravity: Analog pH Sensor/Meter Pro Kit for Arduino is a professional Arduino pH Sensor Meter Kit with an industrial electrode. It has built-in simple, convenient, practical connection and long life (up to 1 year), which makes it very suitable for long term online monitoring. It has an LED which works as the Power Indicator, a BNC connector, and a PH2.0 sensor interface. To use it, just connect the pH sensor with the BND connector, then plug the PH2.0 interface into the analog input port of any Arduino controller.
























 Labdisc
Labdisc Botzees
Botzees Edison
Edison Telepresence Robot
Telepresence Robot DOBOT
DOBOT Keyestudio
Keyestudio Fischertechnik
Fischertechnik